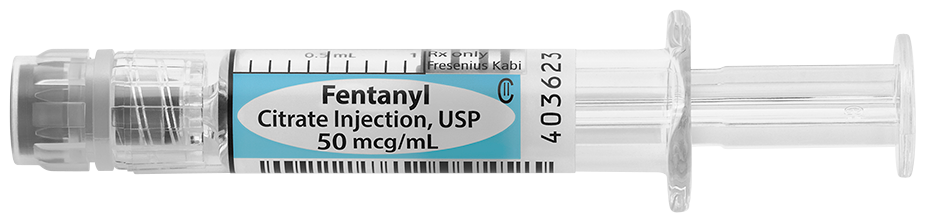
Fentanyl Citrate Injection, for intravenous or intramuscular use, is indicated for:
- Analgesic action of short duration during the anesthetic periods, premedication, induction and maintenance and in the immediate postoperative period (recovery room) as the need arises.
- Use as an opioid analgesic supplement in general or regional anesthesia.
- Administration with a neuroleptic as an anesthetic premedication, for the induction of anesthesia and as an adjunct in the maintenance of general and regional anesthesia.
- Use as an anesthetic agent with oxygen in selected high risk patients, such as those undergoing open heart surgery or certain complicated neurological or orthopedic procedures.
Fentanyl Citrate Injection should be administered only by persons specifically trained in the use of intravenous anesthetics and management of the respiratory effects of potent opioids. Ensure that an opioid antagonist, resuscitative and intubation equipment, and oxygen are readily available.
Fentanyl Citrate Injection is contraindicated in patients with a hypersensitivity to fentanyl.
Risks of Skeletal Muscle Rigidity and Skeletal Muscle Movement: Manage with neuromuscular blocking agent. See full prescribing information for more detail on managing these risks.
Severe Cardiovascular Depression: Monitor during dosage initiation and titration.
Serotonin Syndrome: Potentially life-threatening condition could result from concomitant serotonergic drug administration. Discontinue Fentanyl Citrate Injection if serotonin syndrome is suspected.
Adrenal Insufficiency: If diagnosed, treat with physiologic replacement of corticosteroids, and wean patient off of the opioid.
Risks of Use in Patients with Increased Intracranial Pressure, Brain Tumors, or Head Injury: Monitor for sedation and respiratory depression.
The most common serious adverse reactions were respiratory depression, apnea, rigidity, and bradycardia.
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176, option 5, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Concomitant Use of CNS Depressants: May decrease pulmonary arterial pressure and may cause hypotension. See full prescribing information for management instructions. For post-operative pain, start with the lowest effective dosage and monitor for potentiation of CNS depressant effects.
Mixed Agonist/Antagonist and Partial Agonist Opioid Analgesics: Avoid use with Fentanyl Citrate Injection because they may reduce the analgesic effect of Fentanyl Citrate Injection or precipitate withdrawal symptoms.
Pregnancy: May cause fetal harm.
Lactation: Infants exposed to Fentanyl Citrate Injection through breast milk should be monitored for excess sedation and respiratory depression.
Geriatric Patients: Titrate slowly and monitor for CNS and respiratory depression.
This Important Safety Information does not include all the information needed to use FENTANYL CITRATE safely and effectively. Please see full prescribing information, including BOXED WARNING, for FENTANYL CITRATE INJECTION.