Minimize risk by reducing diversion opportunities
Reduce waste
As healthcare costs continue to rise, it is essential to limit medication waste and workforce time waste whenever and wherever possible.1
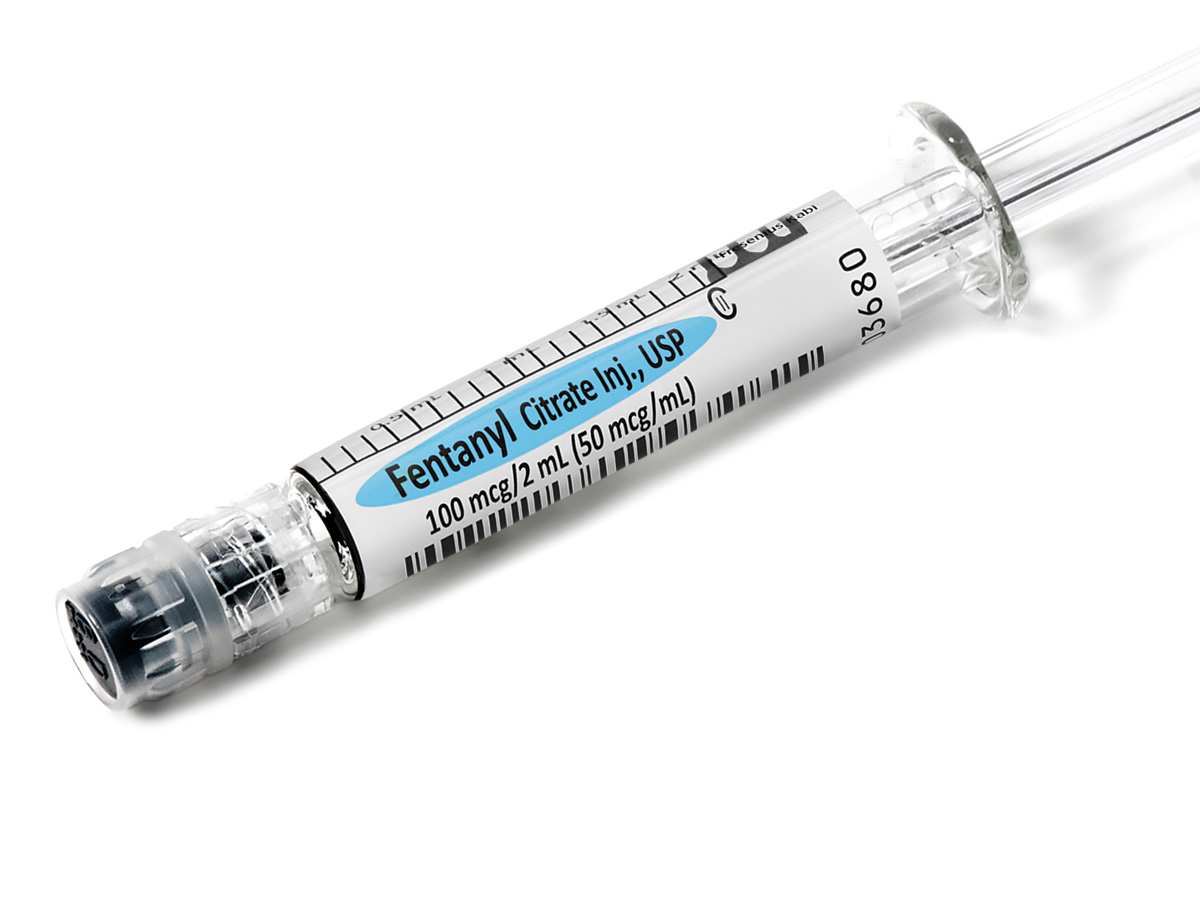
Simplist Fentanyl 50 mcg/mL in a 1 mL presentation helps enable optimization of product size to common practice.
“Limiting product waste and related documentation in the current environment is critical. Eliminating waste helps to streamline operations and free up staff time.“
Nathan Wooten, PharmD, Director of Pharmacy, United Regional Health Care System
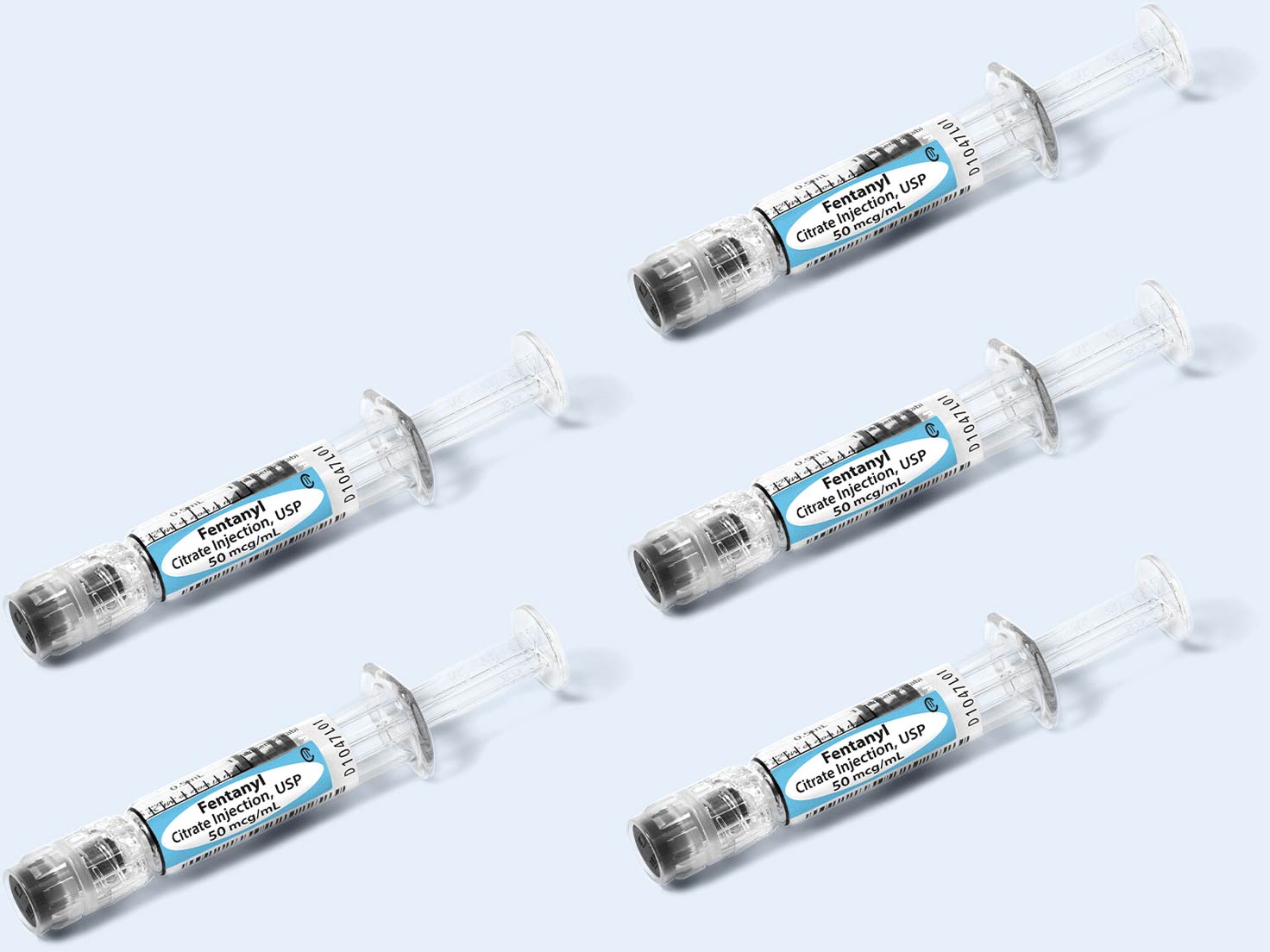
Reduce diversion opportunities
Opioid abuse has reached epidemic proportions, and fentanyl, one of the most potent opioids, is the most commonly diverted drug.2
Given the high abuse potential of fentanyl, it is imperative to minimize product waste and secure product dispensing to help reduce potential diversion.
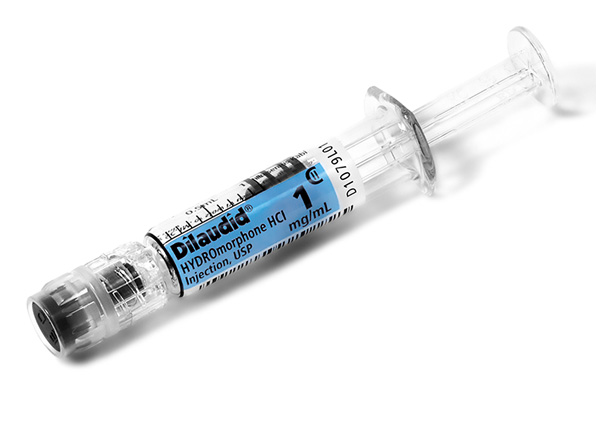
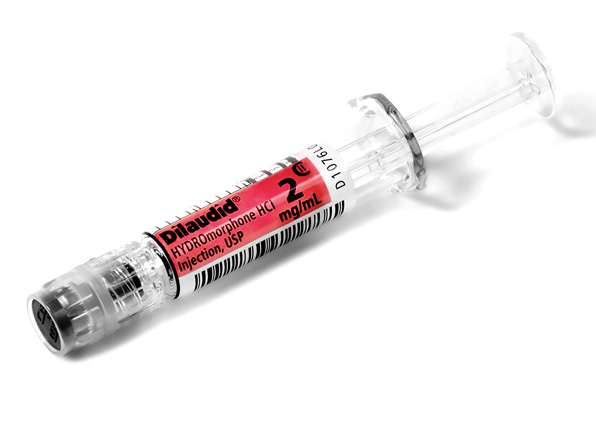
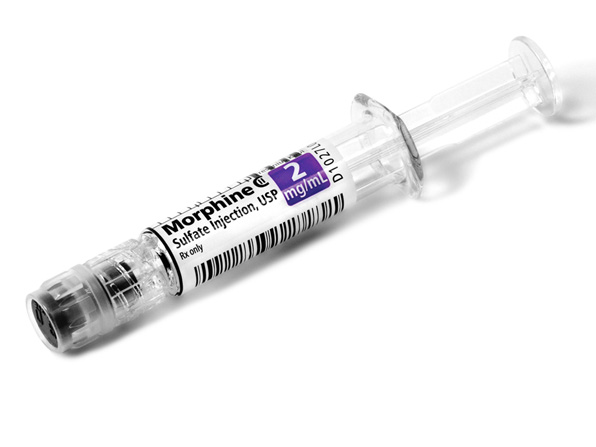
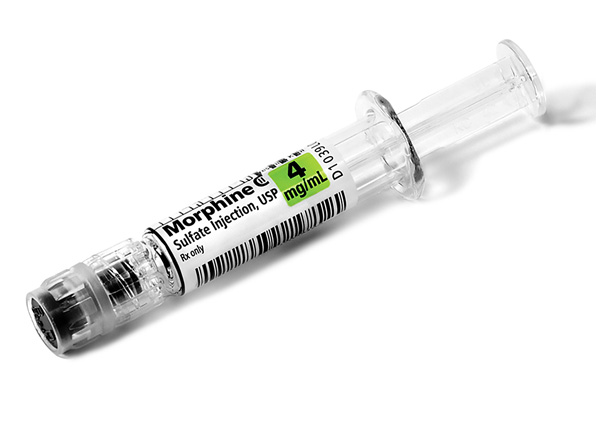
Simplist MicroVault packaging supports secure dispensing with a rigid, transparent polypropylene shell and tamper-evident seal and makes narcotic inventory management easier with 5-pack bundling and 10-pack cartons.
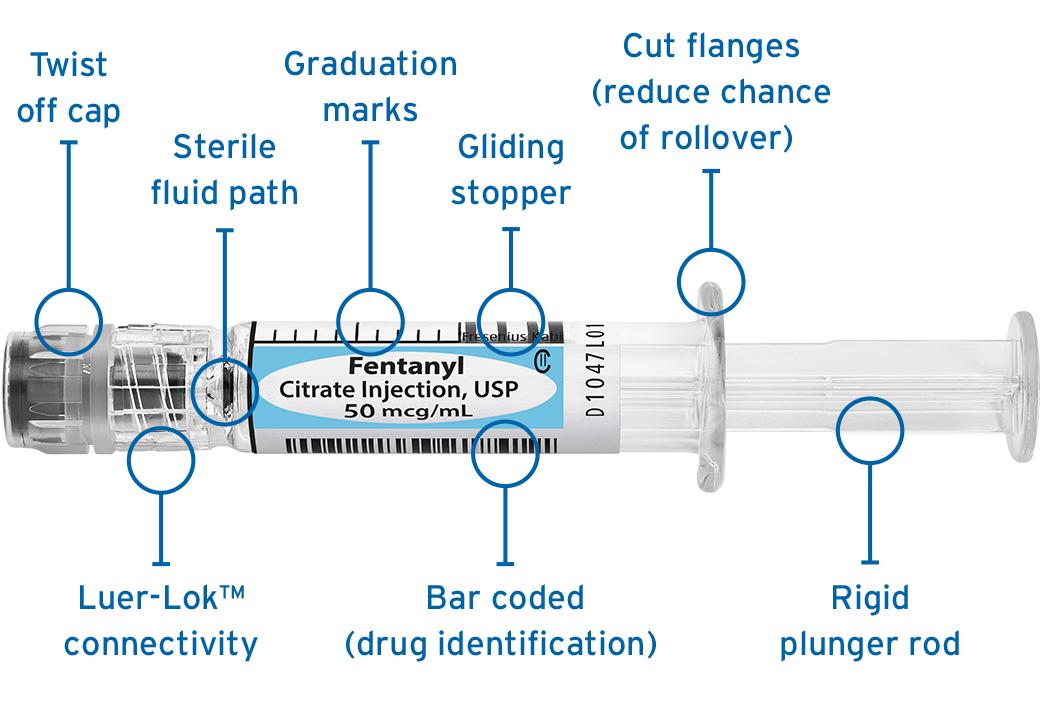
Ready-to-administer (RTA)
Simplist ready-to-administer prefilled syringes streamline point-of-care preparation with no assembly required and are ready to go.
- Single-unit dose
- 24- or 36-month shelf life
- cGMP + FDA-approved
- Clear and distinct labeling
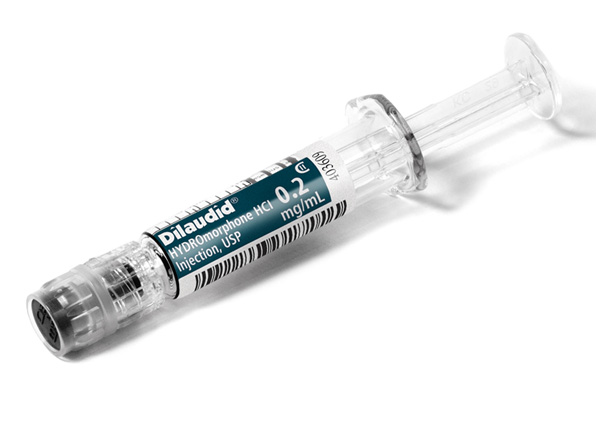
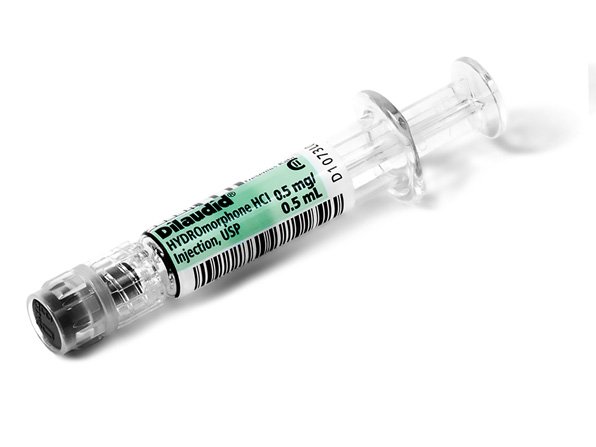
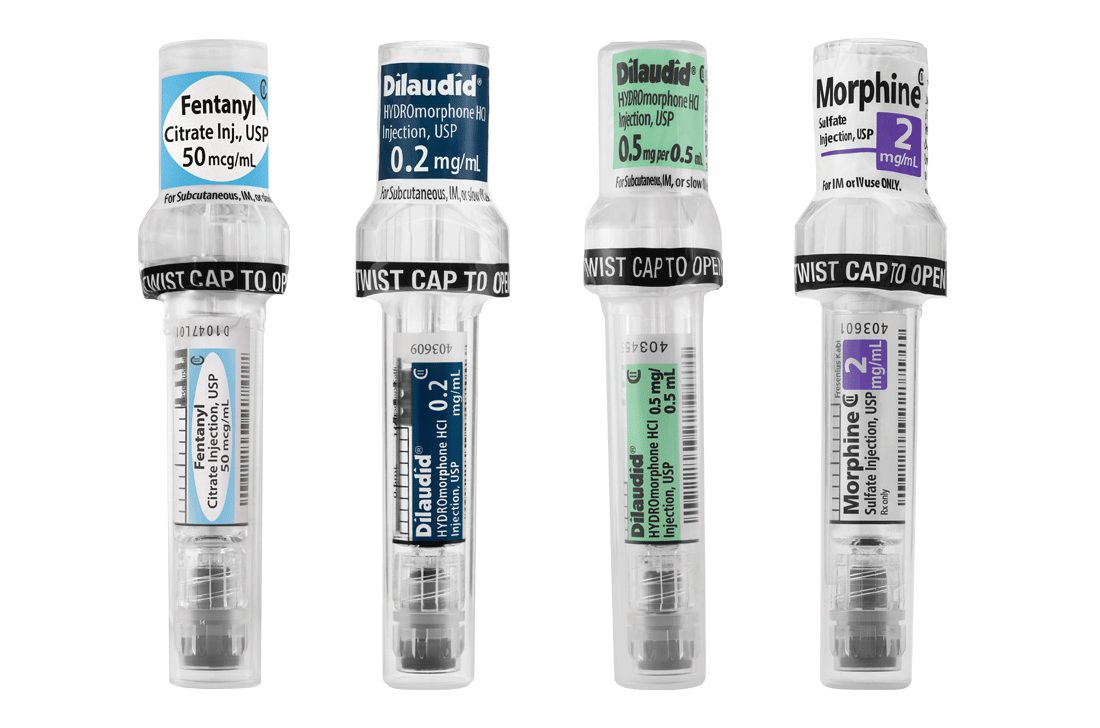
Comprehensive Narcotic Portfolio
Simplist prefilled syringes in MicroVault tamper-evident packaging provides options that may help enable product size optimization in a consistent drug delivery system across our portfolio.
References
The Third Consensus Conference on the Safety of Intravenous Drug Delivery Systems was convened to evaluate the benefits and risks of available systems and assess ongoing threats to the safety of intravenous drug delivery.*
The Third Consensus Conference on the Safety of Intravenous Drug Delivery Systems was convened to evaluate the benefits and risks of available systems and assess ongoing threats to the safety of intravenous drug delivery.*
The purpose of these guidelines is to provide guidance to health systems on planning for and implementing best practices when establishing a comprehensive Controlled Substance Diversion Prevention Program.
The purpose of these guidelines is to provide guidance to health systems on planning for and implementing best practices when establishing a comprehensive Controlled Substance Diversion Prevention Program.
This article discusses the financially significant costs associated with wasting both the product and the valuable time of a skilled workforce and ways to reduce this financial burden on our health-systems.*
This article discusses the financially significant costs associated with wasting both the product and the valuable time of a skilled workforce and ways to reduce this financial burden on our health-systems.*
This study suggests that careful selection of vial size has the potential to modify perioperative dosing practices and possibly reduce opioid use.
This study suggests that careful selection of vial size has the potential to modify perioperative dosing practices and possibly reduce opioid use.